Proton-detected experiments
The 1H nucleus is characterized by high sensitivity, high natural abundance and a ubiquitous presence. This makes the proton an attractive nucleus for structural studies also in solid-state NMR. On the other side, the dominant homonuclear dipolar interactions usually lead in solids to broad 1H resonances, which obscure all details in the spectrum such as the chemical-shift information.
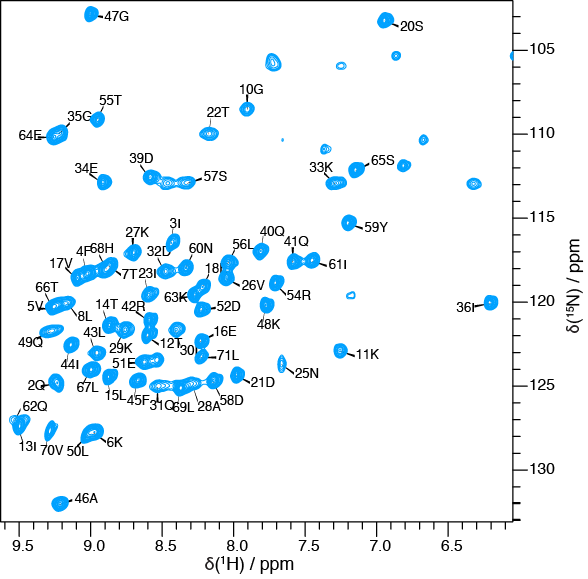
Fast MAS and very high static magnetic fields allow the implementation of experiments based on 1H detection which, in combination with extensive degree of deuteration in proteins, can increase coherence lifetimes and spectral resolution in 1H solid-state NMR. We have been working at the development of pulse sequences based on proton detection in partially deuterated proteins.
Recently, we have shown that employing proton-detected NMR experiments at 100 kHz MAS it is possible to determine the de novo 3D structure from sub-milligram protein samples.
Collaboration with external page Anja Böckmann (IBCP Lyon, France), external page Ago Samoson (Tallinn University of Technology, Estonia) and external page Vipin Agarwal (TIFR Hyderabad, India).